FDA Vaccine Advisory Committee Votes to Recommend Booster Vaccines with Omicron Component
On Tuesday, June 28, 2022, the Food and Drug Administration’s (FDA) Vaccine and Related Biological Products Advisory Committee (VRBPAC) met to discuss and vote on whether to recommend an Omicron component in COVID-19 booster vaccines.
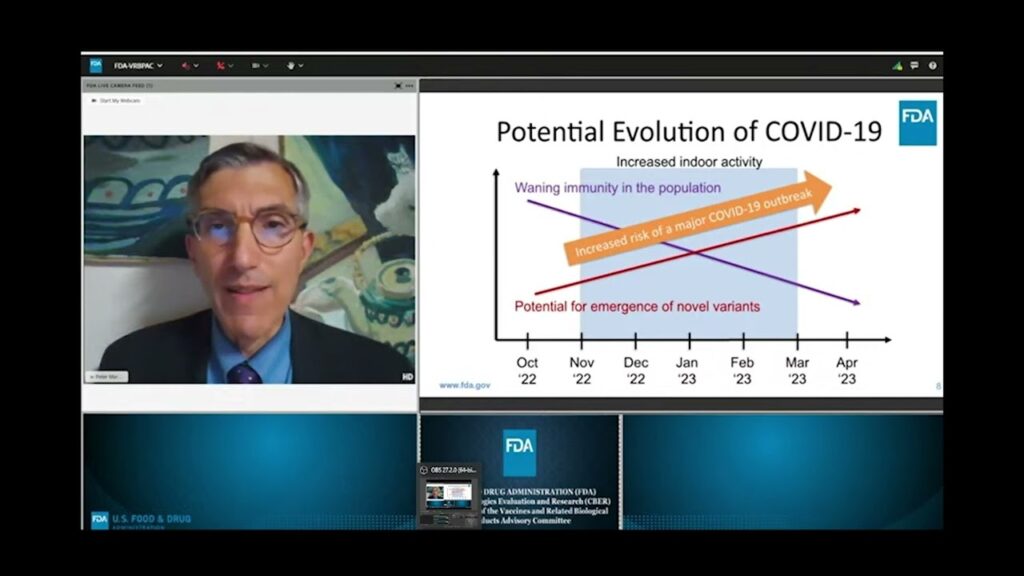
Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research told the committee that the “combination of waning immunity combined with the potential immergence of novel variants during a time this winter when we will move inside as a population increases our risk of a major COVID-19 outbreak, and for that reason we have to give serious consideration to a booster campaign this fall to help protect us during this period from another COVID-19 surge.”
VRBPAC members voted 19-2 in favor of recommending the inclusion of a SARS-CoV-2 Omicron component for COVID-19 booster vaccines in the United States.
Video source: FDA
source
